Abstract
Background: Despite initial responses to standard chemotherapy, most patients with acute myeloid leukemia (AML) relapse and have poor prognoses after subsequent therapy. The hypoxic bone marrow (BM) microenvironment is hypothesized to contribute to clinical resistance through the sheltering of AML blasts and leukemia stem cells (LSCs), allowing them to evade chemotherapy and reinitiate the disease. We have previously shown that AML cell lines cultured under prolonged hypoxia (1% O2) can upregulate autophagy and become resistant to cytarabine (AraC). Furthermore, the inhibition of autophagosome turnover with either Bafilomycin A1 (Baf A1) or chloroquine (CQ) can re-sensitize AML cells to AraC. Additionally, Baf A1 therapy effectively eradicated in vivo functional LSCs in a primary human AML xenotransplantation mouse model. However, clinical development of BafA1 and CQ has been limited by poor drug pharmokinetics. Lys05 is a novel water-soluble CQ derivative which exhibits 10 fold more potent autophagy inhibitory properties than CQ in vitro and is well tolerated in mouse models. Here, we tested the effects of Lys05 on human AML growth, chemoresistance under hypoxia, and in vivo leukemia burden in a human AML xenograft model. We also explored the effects of autophagosome accumulation on cellular metabolism as a new mechanism for targeting chemoresistant AML blasts and LSCs.
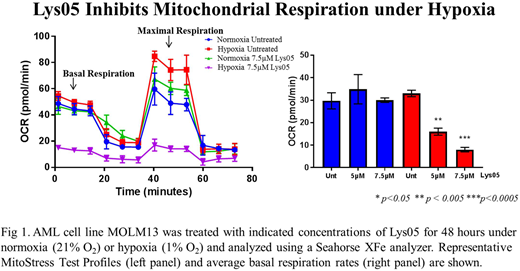
Methods: Human AML cell line MOLM13 and CD34 positive AML patient samples were cultured under hypoxia (1% O2) or normoxia (21% O2) with cytarabine (AraC) and late-stage autophagy inhibitor Lys05 alone and in combination. After 72 hours of drug exposure, apoptosis was measured using Annexin V/propidium iodide flow cytometry. Metabolic assays were performed using a Seahorse XFe96 analyzer to measure oxygen consumption and extracellular acidification rates using the Mitochondrial or Glycolytic Stress Tests on AML cells treated with late-stage autophagy inhibitors for 48 hours. Mitochondrial mass was measured following staining with MITO-ID Green Detection Kit using flow cytometry. Human xenograft models were performed using NSG mice inoculated with MOLM13 BLIV, a luciferase-expressing human AML cell line, and treated with either vehicle or Lys05 (20 mg/kg 4 days on/3 off for 4 weeks). Tumor burden was assessed using bioluminescence and analyzed with IVIS Living Image Software.
Results: Treatment of AML MOLM13 cells with Lys05 in vitro resulted in a dose-dependent decrease in cell proliferation and increased apoptosis. Lys05 treatment further displayed similar results to BafA1 and CQ, enhancing the anti-leukemic effects of AraC and overcoming hypoxia-induced chemoresistance. Seahorse XFe analysis revealed that BafA1 inhibited both basal and maximal mitochondrial respiration under both normoxic and hypoxic conditions at 24 hours. CQ and Lys05, however, had no effect on oxidative phosphorylation under normoxia, yet significantly decreased mitochondrial function under hypoxia. Interestingly, reduced function did not coincide with a decrease in mitochondrial mass, but rather increased mitochondrial mass. We also observed decreases in glycolytic function after treatment with late-stage autophagy inhibitors under hypoxia suggesting these cells may be undergoing an energy crisis. Treatment of leukemia-bearing mice with Lys05 trended toward decreased tumor burden compared to vehicle. Studies optimizing Lys05 monotherapy and testing combination treatment with AraC are underway.
Conclusions: Our research shows that CQ derivative Lys05 exhibits significant anti-leukemic activity against human AML cells and restores chemosensitivity to cytarabine under hypoxia, further supporting our hypothesis that autophagosome accumulation can overcome hypoxia-induced chemoresistance. We also demonstrate that Lys05 may effectively inhibit leukemia progression in vivo. Our studies further reveal metabolic disruption through the accumulation of autophagosomes as a mechanism for restoring chemosensitivity of AML cells under hypoxia and targeting LSCs. These results establish late-stage autophagy inhibitors, specifically Lys05, as a promising new treatment approach for resistant AML and supports further study of these drugs for treatment of minimal residual disease.
Guzman:Cellectis: Research Funding. Wang:Novartis: Speakers Bureau; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; Amgen: Consultancy; Jazz: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal